US Medical Innovations, LLC (USMI) announced today it has received FDA 510k Clearance (K240297) for the Canady Helios Cold Plasma™ (CHCP) Ablation System for the ablation of soft tissue during surgery. The CHCP system consists of the Canady Helios Cold Plasma XL-1000 CP Smart Electrosurgical Generator, Canady Helios Cold Plasma Ablators, Foot Pedal and Trolley Cart.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240507587574/en/
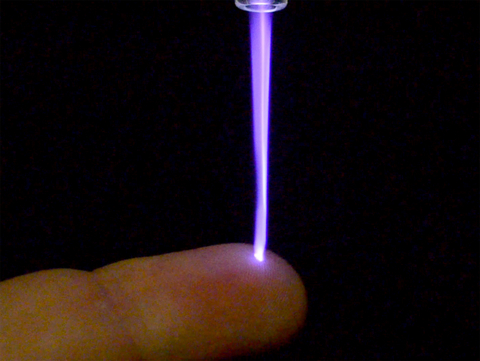
Canady Helios Cold Plasma. (Photo: Business Wire)
Plasma is the fourth state of matter formed by ionizing neutral gases (i.e. Argon or Helium) combined with electromagnetic fields. The Canady Helios Cold Plasma™ System introduces a novel approach to soft tissue ablation using plasma. The system creates a plasma jet consisting of a pre-programmed, pulsed, non-contact, non-thermal (24 C to 30 C), three-dimensional, Plasma Treated Electromagnetic Field™ (PTEF). This plasma jet is applied for 5 to 7 minutes intra-operatively to the microscopic soft tissue surgical margin following the surgical removal of a solid tumor. The PTEF consists of electronically charge particles comprised of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that permeate the cellular membrane via an Irreversible Electroporation process resulting in apoptosis (cell death) without affecting surrounding healthy tissue.
The Jerome Canady Research Institute for Advanced Biological and Technological Sciences (JCRI-ABTS) team previously reported the first Phase I Clinical Trial (IDE #G190165) for Cold Atmospheric Clinical Trial for the Treatment of Advancement of Solid Tumors1. The technology has demonstrated exceptional safety and efficacy profiles with a non-local recurrence rate of up to 80% and an overall survival rate of up to 86%.
Taisen Zhuang, PhD, Chief Technology Officer stated, “This milestone allows us to integrate our revolutionary technology with our vision of creating the world’s first AI-driven cold plasma robotic delivery system. We believe this powerful combination represents the most promising path forward in surgical oncology, offering unprecedented precision and efficacy. Our commitment to pushing the boundaries of medical science and technology has brought us to this pivotal moment, and we are proud to be at the forefront of this transformative era in healthcare.”
Saravana R.K. Murthy, PhD, Vice President of Research at JCRI, highlighted the potential systemic effects of CHCP treatment, stating, “In addition to the advantages associated with surgical margin ablation, our preclinical investigations, supplemented by a multitude of published studies, indicate that CHCP-treated cancerous cells elicit the release of highly specific and immuno-stable antigens, potentially inducing a systemic response conducive to targeted immunotherapeutic applications or as complementary modalities alongside established standards of care.”
Jerome Canady, MD, FACS, CEO and Co-Founder, Surgical Oncologist stated, “This is a very exciting time for our company. We have been working very hard towards this milestone for fourteen years. We are very proud of this remarkable achievement and feel confident that this disruptive technology will help the advancement of medicine and patient care. Special thanks to the Compassionate Use and Phase 1 Patients participant and their families who led the way and the Multi-disciplinary team of Physicists, Engineers, Translational Molecular Scientists, Clinical Researchers and Michael Keidar, PhD (George Washington University), the late Barry Trink, PhD (Johns Hopkins University), Alexey Shashurin, PhD (Purdue University), Giacomo Basadonna , MD, PhD, FACS (University of Massachusetts, Worcester), Steven Gitelis, MD and Keith Millikan, MD, FACS of Rush University Medical Center (RMC), Aviram Nissan, MD and Mohammed Adileh, MD of Sheba Medical Center (SMC) as well as the surgical teams at RMC in Chicago, IL, USA, and SMC in Tel HaShomer, Israel performing these high risk surgical procedures and our research teams Plasma Medicine Engineers, and the JCRI’s researcher for their years of hard work and dedication.”
The Company plans to distribute the Canady Helios™ Cold Plasma System in Hospitals in late 2024.
For more information on US Medical Innovation’s products and technology please visit: www.usmedinnovations.com.
About US Medical Innovations
US Medical Innovations, LLC (USMI), based out of Takoma Park, MD, is a privately held FDA registered life science and biomedical device company. USMI is dedicated to expanding the boundaries of plasma medicine by pioneering new technologies for the development of state-of-the-art medical devices that advance patient outcomes and improve human lives.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240507587574/en/