Multiply Labs, a robotics company developing industry-leading automated manufacturing systems to produce individualized drugs, today announced performance results for its proof-of-concept robotic system, developed along with UCSF, Cytiva, Thermo Fisher Scientific and Charles River Laboratories. This data demonstrates that automated cell therapy manufacturing outcomes (cell quantity and quality) using Multiply Labs’ proof-of-concept robotic system are statistically equivalent to that of a process performed manually. Given this robotic technology is compatible with leading cell therapy manufacturing instruments, the data indicates that it is possible to automate an existing cell expansion protocol without significantly changing the process or impacting product characteristics.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240103978637/en/
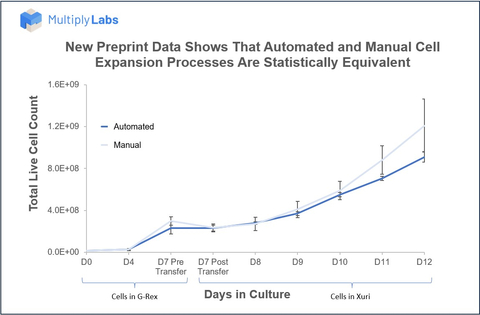
(Graphic: Multiply Labs)
“We are so excited by this initial data as it opens the door to accelerating the availability of cell therapies,” said Fred Parietti, Ph.D., Co-founder and CEO of Multiply Labs. “This data demonstrates that manufacturers can confidently automate their existing processes for cell expansion, without making significant modifications to the process itself, effectively minimizing bioprocess and regulatory risks. With this level of automation there is great potential to decrease labor costs and increase manufacturing throughput for these life-saving therapies.”
To assess the robotic system’s ability to culture T cells, manual and robotic conditions were tested in parallel with three replicates each. For both conditions, human T cells were activated and placed into a small-scale bioreactor in a cell culture incubator. After seven days, T cells were transferred to a large-scale cell expansion system, where they continued to grow for an additional five days. Total cell number and cell viability before bioreactor transfer (day 7) and after harvesting (day 12) were found to be statistically indistinguishable between robotic and manual versions of this culture process. In both conditions, cell yields were greater than live cells and viability remained above 95%. To ensure the maintenance of cell quality, gene expression analyses were performed and no statistical differences were observed in differentiation, exhaustion, and proliferation markers between the manual and robotic conditions. In fact, only 0.45% of approximately 28,000 genes analyzed were differentially expressed between the two conditions.
In addition to cell count and viability successes, none of the robotic cell expansion samples were contaminated. However, one of the three manual T cell expansions was positive to S. epidermidis. This organism is found on human skin and supports the conclusion that removing human hands from manufacturing processes can help prevent microbial contamination.
In summary, the prototype robotic cluster demonstrated comparable cell yields, viability, and identity when compared to manually cultured cells. This is a critical finding for the scalability and availability of cell therapies, given approximately half of cell therapy manufacturing costs are driven by labor and limitations on skilled labor. The high cost of personnel coupled with necessary facility and equipment expenses have contributed to the very high prices for cell therapy and therefore limited patient access.
While robotics and automation are common approaches to reducing labor costs in other industries, it is more challenging in this space as changing instruments or processes in FDA-approved therapeutics manufacturing requires regulatory resubmissions and comparability studies. This is why Multiply Labs’ proof of concept and unique approach focuses on robotic systems that can operate market-leading GMP instruments from multiple different vendors, which are already extensively deployed for cell and gene therapy manufacturing. This enables plug-and-play-like capabilities, and fewer regulatory barriers, as no major process changes are required for robotic compatibility. While this proof of concept focused on cell expansion, there is promise in applying the same approach to cover the whole end-to-end cell therapy manufacturing process by automating more instruments in a similar way.
The research, titled “Development of a robotic cluster for automated and scalable cell therapy manufacturing,” is currently under review. To read the full preprint, click here.
About Multiply Labs
Multiply Labs is a robotics company that provides autonomous manufacturing technology to the pharmaceutical industry. The company develops advanced, cloud-controlled robotic systems that enable the production of individualized drugs at scale. Its customers include some of the largest global organizations in the advanced pharmaceutical manufacturing space. Multiply Labs’ expertise is at the intersection of robotics and biopharma – its team includes mechanical engineers, electrical engineers, computer scientists, software engineers and pharmaceutical scientists. The founding team got in touch because of their shared love of robots at MIT. The company is based in San Francisco, California. For more information, please visit www.multiplylabs.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240103978637/en/