Medical device and technology company Lazurite® announced that a new article just published in the Journal of Orthopaedic Experience & Innovation provides key support for the usability of the company’s ArthroFree® wireless surgical camera.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240125564430/en/
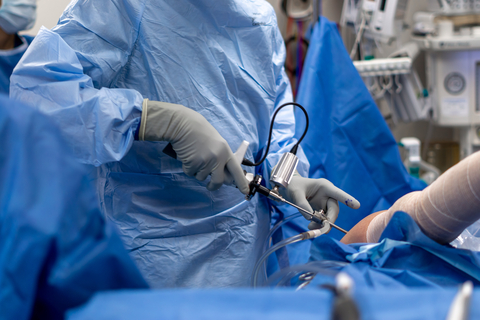
The ArthroFree wireless camera is designed to allow surgeons to operate with optimal dexterity and surgical ergonomics with a focus on the patient’s safety. (Photo: Business Wire)
In a human factors usability analysis of the camera system involving 88 participants, 94.32% of respondents gave a “good” or “excellent” response to the statement, “The absence of cables will have a positive impact on efficiency,” and 90.91% gave a “good” or “excellent” response to the statement, “The absence of cables will have a positive impact on patient safety.” More than 88% (88.10%) agreed with the statement, “I could perform surgery with the same degree of confidence [as my current device].”
Separate one-on-one interviews with surgeons on their independent experience with ArthroFree pointed to “untethered movement” as the most important benefit of using the device.
The article, by Pearl Griffin, MS, Patrick Polito, MS, Leah Brownlee, JD, Jeff Ustin, MD, Kipum Lee, PhD, and Craig Connor MS, is available here on the Journal’s website.
ArthroFree is the first FDA-cleared wireless camera for arthroscopy and general endoscopy, both areas of minimally invasive surgery. The device is an alternative to the conventional camera used in minimally invasive surgery, which has two cables, one for power and one for fiber-optic light, tethering it to instrumentation boxes on a surgical tower. The cables can be cumbersome, routed around or across the patient, and risk bridging the sterile and nonsterile fields.
Feedback on the ArthroFree camera system’s usability was received from 82 surgeons and six other medical professionals. Topics surveyed included ergonomics, ease of use, image quality, and patient experience. Surveys were collected following design validation testing (pre-FDA clearance; 76 participants) or clinical evaluation (post-FDA clearance; 12 participants).
Participants with wide-ranging levels of experience in minimally invasive surgery were recruited from 10 medical facilities. They were trained on the use of ArthroFree and then used the device in a surgical procedure performed on a patient or model. Their experience was evaluated in a 13-statement human factors survey scored using a 5-point Likert scale. Participants scored the device’s usability “good” or “excellent” in all survey statements, suggesting users’ needs were satisfied in 91.30% of participants.
To complement the usability analysis, one-on-one interviews were conducted with 15 surgeons from nine medical facilities gathering qualitative feedback on their independent experience with the ArthroFree device. Each surgeon was asked to comment on the potential benefits of using the device. All 15 volunteered “untethered movement” as an expected benefit. Other benefits cited by more than half of those interviewed include decreased setup and teardown time (11/15), and increased efficiency in the OR (8/15).
“The ArthroFree Wireless Camera System is designed to free the surgeon from the tethers of video power and fiber-optic light cables in order to allow more ergonomic and efficient surgical movements and enhance the safety of the OR,” said Dr. Jeff Ustin, a critical care surgery specialist at University Hospitals and Akron General Medical Center. “The favorable survey scores and benefits expressed by surgeons in this analysis support the usability of the first wireless surgical camera and a high likelihood of user adoption.”
“The publication of the usability analysis is the culmination of the initial phase of our collaboration with Lazurite in support of its ArthroFree wireless camera system,” said Kipum Lee, vice president of innovation & product strategy at UH Ventures, the innovation and commercialization arm of University Hospitals. “The usability analysis represented a great opportunity for our surgeons and surgical teams to use the device. This type of immersive observation and data gathering is one of the key engagement services we provide through our UH Ventures platform.”
For more information about the ArthroFree System, contact a product expert at (833) 214-2324 or contact@lazurite.co.
About the Authors
Pearl Griffin is the senior manager of research & evidence generation at Lazurite. She holds a Master of Science in Biology from the University of Rochester. Prior to joining Lazurite in 2022, she worked for 10 years at Buffalo Biolabs, most recently as president & managing director, and as senior program manager with Cleveland BioLabs concentrated on government contracting.
Patrick Polito is the director of regulatory & compliance at Lazurite. He received a Master of Science in Microbiology from SUNY Brockport. Prior to joining Lazurite in 2016, he spent 11 years working in medical device sterilization, testing, and reprocessing at Iuvo BioScience (previously Moog), where he held various roles including business development, facility and project management, quality and regulatory assurance, and directing/performing laboratory science. He sits on 15 American National Standards and Technical Information Report standards committees and working groups.
Leah Brownlee is president and general counsel at Lazurite, receiving her Doctor of Law from Case Western Reserve University (CWRU). She joined Lazurite in her current roles in October 2020 and serves as board chair of Switch Automation (US), Inc. Her previous positions include of counsel in the global corporate department at Squire Patton Boggs (US) LLP, a top-20 global law firm, and executive vice president of compliance and operations at Cleveland Biolabs.
Jeff Ustin is a critical care surgery specialist in Cleveland with more than 25 years of experience. He is affiliated with University Hospitals (UH) Rainbow Babies & Children’s Hospital and Akron General Medical Center and is an assistant professor at CWRU School of Medicine. He graduated from Stanford University Medical School. He completed his residency in General Surgery at UH followed by a research fellowship in General Surgery. He also completed a residency in General Surgery at Emory University Hospital and a fellowship in Trauma and Acute Care Surgery at Massachusetts General Hospital.
Kipum (Kip) Lee is vice president of innovation & product strategy at UH. He received a Master of Design in Interaction Design from Carnegie Mellon University, and a PhD in Management from CWRU. He is also an assistant professor in the CWRU School of Medicine’s Department of Medicine at UH Cleveland Medical Center and an assistant professor in the Department of Design & Innovation at the CWRU Weatherhead School of Management.
Craig Connor has recently joined the University of Wisconsin (Madison) as an adjunct assistant professor in the Mechanical Engineering Department. Prior to this position, he was director of human factors in the Madison studio of the human factors engineering firm Delve. He received a Master of Science in Mechanical Engineering with a Design & Ergonomics emphasis from the University of Wisconsin.
About Lazurite
Lazurite designs medtech devices. Its ArthroFree® System includes the first wireless camera with FDA clearance for arthroscopy and endoscopy. Free of cumbersome camera cables, ArthroFree enables surgeons to work with maximum dexterity, precision, and focus. Without camera-cable-related interruptions and contamination concerns, surgeons can keep their eyes on the monitor—and on their patients’ well-being. Lazurite is located in Cleveland, OH (est. 2015). Its investors include more than 70 physician champions. Lazurite’s IP portfolio includes the high-efficiency Meridiem® light technology, wireless surgical camera technology, and products in development. Lazurite’s three-year vision (by 2026): Modernize 1,000 operating rooms for the wireless age. For more information, see https://lazurite.co.
Forward-Looking Statements
This press release includes “forward-looking statements.” Forward-looking statements can be identified by words such as: “anticipate,” “intend,” “plan,” “believe,” “projected,” “estimate,” “expect,” “may,” “should,” “will,” “designed,” “optimistic,” “promises,” “helps,” “allows” and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding the expected impact and ease of use advantages of the ArthroFree System within the field of minimally invasive surgery, particularly orthopedic surgery. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations, and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy, and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Any forward-looking statement made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Lazurite®, ArthroFree®, and Meridiem® are registered trademarks of Lazurite Inc.
Media Center
Visit lazurite.co/media for videos, logos, images, fact sheets, bios and more.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240125564430/en/