Cyrus Biotechnology, Inc., today announced data demonstrating potent activity for its engineered IgG-degrading enzyme in a rabbit redosing model, with no anti-drug antibodies (ADAs) evident following repeat administration at therapeutic doses.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241211633894/en/
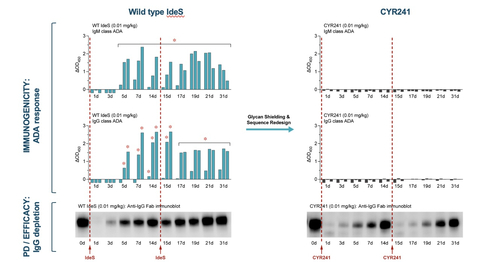
Figure 1. Redosing of CYR241 IgG degrader enzyme at 0.01 mg/kg in rabbit shows no ADAs (right), while WT shows IgM and IgG ADAs (left). (Graphic: Business Wire)
The IgG-degrading enzyme of S. pyogenes (IdeS) has been approved in wild type form for use in certain kidney transplantation recipients, where depletion of the IgG class of antibodies successfully mitigates host immunity against the transplant. Because of this potent IgG degrading capability, WT IdeS has proven broadly potent in pre-clinical studies and is currently in clinical trials in multiple indications including anti-GBM disease and Guillan-Barré Syndrome. However, broader application of IdeS in chronic autoimmune disease has been hampered by the enzyme’s short half-life and a strong anti-IdeS immune response. Cyrus’ IdeS has a significantly extended half-life, elicits no ADAs in a relevant animal model and demonstrates potent redosability in vivo.
CYR241, a Cyrus IgG degrader, does not elicit ADAs even after redose, unlike WT IdeS which elicits a strong ADA response after the first dose. In this new study, the WT IdeS control and CYR241 were dosed at 0 and 14 days in rabbits (Figure 1). A rabbit preclinical model was used for pharmacokinetic (PK), pharmacodynamic (PD) and immunogenicity evaluations as the enzyme is highly active in humans and rabbits, but not other preclinical species, and rabbits are widely used in vaccine development for comparative assessment of immunogenicity. The WT enzyme elicits strong IgM and IgG ADA responses by 5-7 days post administration in this model, with continued ADA formation after the second dose. CYR241, with near WT levels of activity, demonstrates a complete absence of IgM and IgG ADA responses after a first or a second dose.
CYR241 remains highly potent upon a second dose with similar activity to that of the first dose, while the WT enzyme loses potency (Figure 2). The first WT dose rapidly reduces IgG levels, while the second is much less potent, mirroring results seen in the clinic with the WT enzyme and close variants. In contrast, CYR241 lowers IgG levels even further upon a second dose.
“The IdeS enzyme is the ideal novel product candidate for IgG driven diseases where the anti-FcRn class has established a clear role for IgG reduction as a therapeutic strategy,” said Dr. Tony Manning, Board Director at Cyrus and former CSO at Momenta Therapeutics (Acquired by J&J), where he was the developer of the highest potency anti-FcRn therapeutic, Nipocalimab. “In the autoimmune field we have not had the technology to create a redosable, half-life extended IdeS until Cyrus’s platform.” CYR241 is optimized for glycosylation, which shields the protein from neutralizing antibodies (B cell responses), and its sequence has been reengineered for low MHC binding (T cell activation), all using Cyrus hybrid AI- and screening-based platform to evaluate thousands of MHC/peptide interactions in parallel.
Cyrus will be attending the 2025 JP Morgan conference in San Francisco and rolling out details of this program and deimmunization methods at the PepTalk conference in San Diego in January.
About Cyrus Biotechnology
Cyrus Biotechnology is a Seattle-based pre-clinical-stage AI-driven therapeutics company with an internal pipeline of novel biologics primarily in autoimmune indications. In addition to IdeS, Cyrus is also advancing multiple discovery stage cytokine programs based on years of experience with partners in cytokine optimization. The company was co-founded with Prof. David Baker, 2024 Nobel Prize winner and inventor of Rosetta and numerous protein design AI tools. Cyrus has worked with dozens of Pharma firms on protein redesign for novel therapeutics, including Genentech and Janssen. In 2021 Cyrus started an internal therapeutics pipeline. The company is funded by lead investor Orbimed, with Agent Capital, Hillhouse, Alexandria, and others.
For more information about Cyrus please visit https://cyrusbio.com/
NOTICE: The information contained in this document is dated as of December 11, 2024. Cyrus Biotechnology, Inc. (the Company) disclaims any obligation to update such information after such date. This document contains forward–looking statements reflecting the Company’s current expectations that necessarily involve risks and uncertainties. Actual results and the timing of events may differ materially from those contained in such forward-looking statements due to a number of factors and the Company undertakes no obligation to revise or update any forward-looking statement to reflect events or circumstances after the issuance of this press release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241211633894/en/